Tracking and Evaluation Core

Brian Melancon
Core Director
Pennington Biomedical Research Center
(225) 763-2980
Contact this core
Email: tracking@lacats.org
About This Core
The Tracking and Evaluation (T&E) Core is charged with developing and implementing strategies, processes and activities to collect useful data and provide meaningful evaluations at both the Core-level and overall Center-level. The core is lead out of Pennington Biomedical Research Center.
The goal (specific aim) of this core is to strengthen and oversee the execution of an internal tracking and evaluation plan that ensures ongoing monitoring, informative interim assessments, flexibility to pivot priorities, and an annual evaluation at both the Core-level and Center-wide level for new and continuing initiatives.
Measuring & Evaluating Progress
Formative assessments for the Center are based on OC and Steering Committee discussions, informal phone calls and meetings with the PI, Co-PDs, Core leaders and personnel, and data collected via SPARC, REDCap, PubMed, NIH RePORTER, user feedback as well as the Core level assessments. The T&E team meets with the PI weekly to facilitate a quick feedback loop and identify immediate next steps. Annual summative evaluation includes the independent EAC evaluation. The T&E team will follow the logic model supplemented by the overarching progress indicators. The focus will be on assessing: a) organizational strengths and progress toward achieving the LA CaTS four overarching specific aims and five-year milestones; b) use, quality and costs of programs, resources, and services; budget management, resource allocation, program development or improvement, challenges; c) growth in CT research focused on underserved, vulnerable populations and addressing health disparities; and, d) identifying the results of LA CaTS Center work with the IDeA-CTRs, CTSAs and others to move national goals forward. Emphasis will be placed on assessing new practices or methods initiated by the LA CaTS Center for delivering programs, resources, and services. |
The LA CaTS Center metrics and progress indicators are based on our External Advisory Committee recommendations, the direction of our funder the NIH-NIGMS and the needs of the state as identified by the LA CaTS Center to address health disparities and improve health outcomes. The progress objectives are summarized in the below graphic:
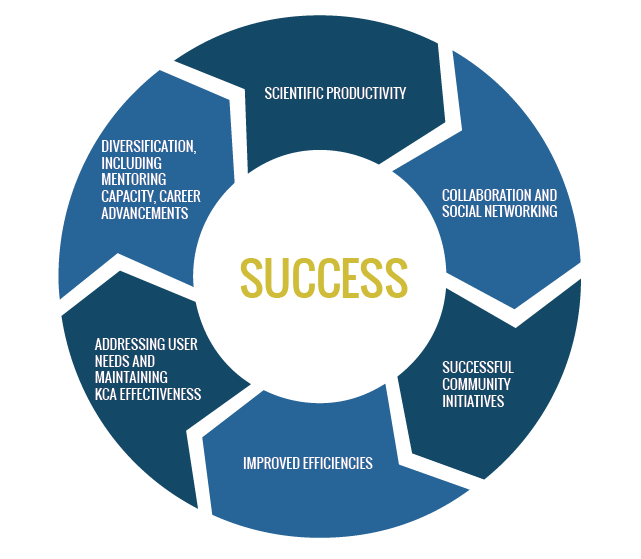
|
Measure #/type of scientific product and other units of related information required Scientific products include manuscripts, publications, presentations, posters, external grant submissions and awards, intellectual property activity and completed scientific advance forms submitted annually to NIH-NIGMS. |
Measures:
- #/type of investigator and study characteristics reported in SPARC (e.g., name, degree, volunteered demographics; study title, disease state(s) or impact area focus; T0-T4 classification);
- CTU activity - #/type/title/name of protocols supported in the CTUs each quarter; #/type, activity of study participants in CTUs each quarter;
- #users/type/results of internal competitive funding/training opportunities;
- #/type of training and/or professional development opportunities that are open to all member institutions and other stakeholders;
- CE Core infrastructure support for research, training, collaborating, and communicating;
- #/type demographics related to diversity member institution faculty, students, personnel using LA CATS resources.
|
Measures:
|
|
Measures:
|
|
Measures:
|
|
Measures:
|
|
Measures:
|
| SPARC is our web-based central portal for requesting services & resources across the LA CaTS Center that was developed by the Medical University of South Carolina CTSA. Valuable information is tracked from our investigators using SPARC. The information gathered is used for NIH reporting, advisory committees, and overall improvements in efficiencies and reach of our investigators we serve. SPARC is a cross-core collaborative effort between the T&E and Cyberinfrastructure Enhancement Cores. See SPARC here. |
This guide combines NIH’s RPPR and NIGMS’s updated reporting guidelines for CTRs.
NIH and NIGMS Reporting GuidelinesThis document was update in July 2024.

